
1715 posts
Location
ACDC Town
Posted 01 June 2016 - 02:33 PM
Over the course of about two days, I cobbled together an interactive, clickable periodic table of elements…for computercraft!
As it requires clicking and colors, advanced computers only.
pastebin get vxLQ1fVb elements
std PB vxLQ1fVb elements
std ld elements <name>
This amazingly organized list of
all things ever looks exactly like the real deal! All organized by group and period, all with their correct atomic masses and whatnot…hell, it gives you the state of matter at 64 degrees F (room temperature)! And everything is labeled by color by state, with a blue box indicating the F-block! What more could you ask for?
Screenshots:
Spoiler
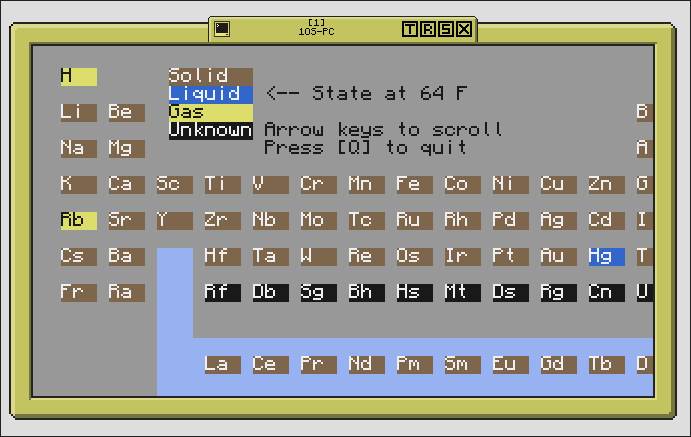
The left half of the table
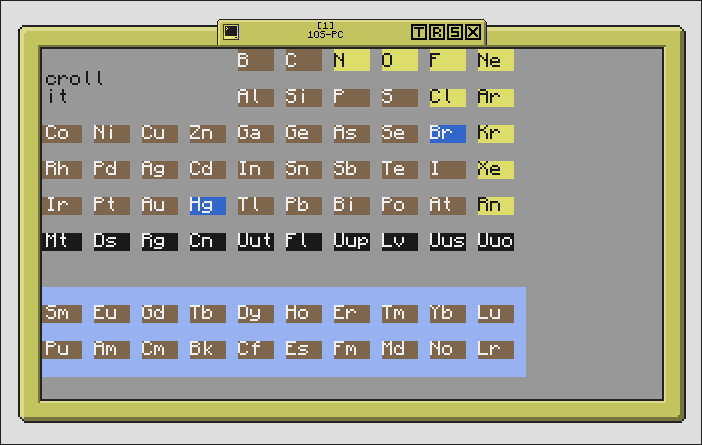
The right half.
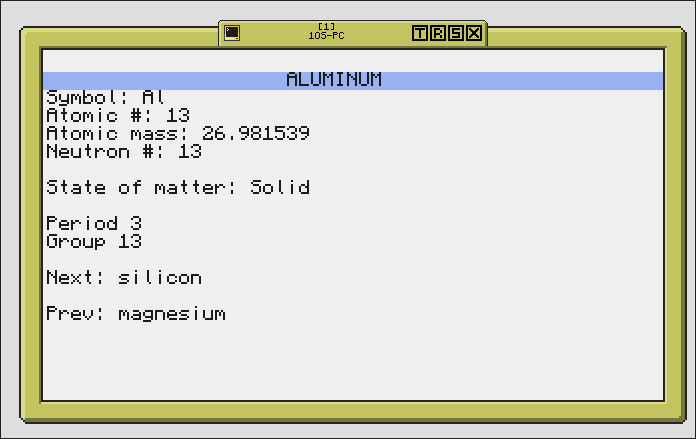
Click on an element to view information on it! From here, you can also press LEFT and RIGHT to go to 'Next' and 'Prev'Edited on 01 June 2016 - 01:39 PM

2679 posts
Location
You will never find me, muhahahahahaha
Posted 01 June 2016 - 04:17 PM
Dude, seriously, no electron configuration?
Just joking, awesome program.

1715 posts
Location
ACDC Town
Posted 01 June 2016 - 08:14 PM
Electron configuration is coming soon! Man did that confuse me in chemistry class.

2679 posts
Location
You will never find me, muhahahahahaha
Posted 01 June 2016 - 09:14 PM
Electron configuration is coming soon! Man did that confuse me in chemistry class.
Man does it still confuse me in chemistry class. Remember, SPDF.
And I actually downloaded the thing and will use it as a reference. (With double checking of course.)

779 posts
Location
Kerbin
Posted 10 June 2016 - 08:35 PM
Time to correct some information:
https://youtu.be/wswa0NuBbMw

1715 posts
Location
ACDC Town
Posted 10 June 2016 - 09:32 PM
Time to correct some information:
https://youtu.be/wswa0NuBbMw
New element names!! Must change!
EDIT: Did change! And I don't feel like putting electron configurations…
Edited on 10 June 2016 - 07:35 PM

135 posts
Posted 30 July 2016 - 08:57 AM
Electron configuration is coming soon! Man did that confuse me in chemistry class.
Electron configuration is a breeze for me if you would like me to help, here's a program i made to calculate configs from atomic number it isn't perfect as
some elements have strange sub-orbital fills for instance Au (Gold) it fills the 3d before 4s when most others fill 4s before 3d (Scientist think it has to do with protons in the nucleus)
Program ->
http://pastebin.com/w1aYywVXP.S before i made this i wasn't aware of the nuclear strong force (don't ask me why lol) but yeah no atom could have 570 electrons because
that would imply that it had 570 protons which would be impossible because the force between the positive charges would explode the nucleus
as the force between just 2 protons is that of 36lbs or (16.3293kg)!!!
Edited on 09 January 2017 - 11:56 PM






